99K
HAI Deaths in the US. A surprisingly little talked about topic in general media.
4M
28.4
Billion dollars cost directly to hospitals caused by HAIs in the United States each year.
3rd
BLUE Connecting Packaging to Patients
Our goal is to turn Hospitals, clinics and surgery centers Blue with our industry leading medical pouch sealers that are the headwater of the SterileAware™ “Medical device packaging system improvement project” These simple to operate rotary sealers have been designed to replace the inadequate low-tech medical pouch sealers that could risk the sterility of your surgical devices. Hawo and Van der Stähl Scientific have collaborated with Sterile Aware® in order to provide a medical pouch sealer that meets the more robust requirements for sterile device packaging. Your engineering group will also benefit from these amazing sealers as preventive maintenance is fast and simple. Why risk a sterility loss on your devices?
Sterile device delivery to patient’s
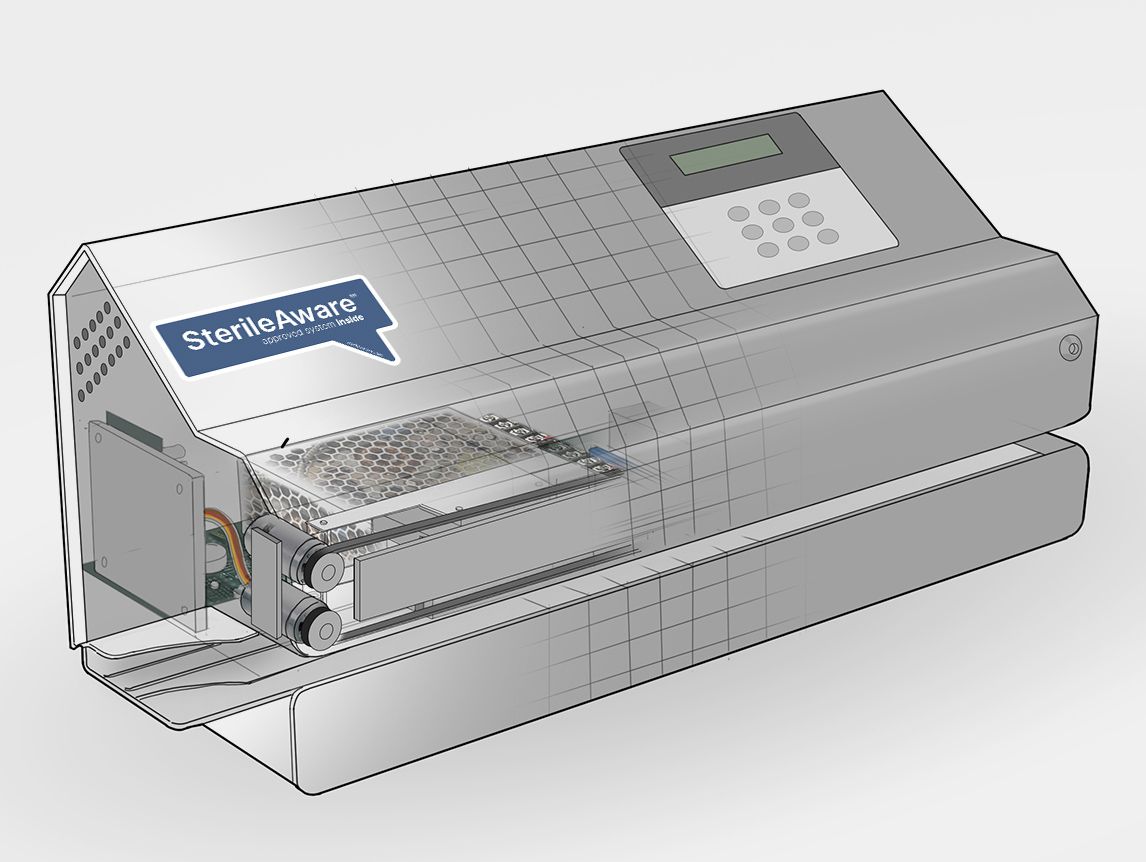
Hawo and Van der Stähl Scientific will power the SterileAware™ “Medical device packaging system improvement project” with state-of-the-industry packaging technology for hospitals, clinics, and MDM’s. And with the SterileAware™ “approved systems inside” campaign, we deliver confidence of conforming packaging systems. Your promise to the patient is the safe and sterile delivery of a medical device to the patient in need. Our feature-rich control system assures all critical sealing parameters of temperature, conveyance speed, and force are compliant with your medical device packaging requirements. The MD-Series “Seal Check” mode verifies system functions for daily process checks.
Supporting advanced technology
BLUE Connecting Packaging to Patients
Our goal is to turn Hospitals, clinics and surgery centers Blue with our industry leading medical pouch sealers that are the headwater of the SterileAware™ “Medical device packaging system improvement project” These simple to operate rotary sealers have been designed to replace the inadequate low-tech medical pouch sealers that could risk the sterility of your surgical devices. Hawo and Van der Stähl Scientific have collaborated with Sterile Aware® in order to provide a medical pouch sealer that meets the more robust requirements for sterile device packaging. Your engineering group will also benefit from these amazing sealers as preventive maintenance is fast and simple. Why risk a sterility loss on your devices?
Sterile device delivery to patient’s
Supporting advanced technology

Our medical device packaging system improvement Project.
-Charlie Webb CPPL (founder)
Category-leading medical
device packaging machines…
The SterileAware™ mission to deliver medical devices safe and sterile to the point of care begins with our partnership with Hawo/Van der Stähl Scientific. This partnership allows us to bring advanced medical device pouch-sealing systems to manufacturers and Hospitals, so they can be assured that their medical devices will reach the point of care safe and sterile. Packaging systems like our feature-rich Nanopak are supporting the healthcare industry’s goal of zero defects on the sterile barrier system.
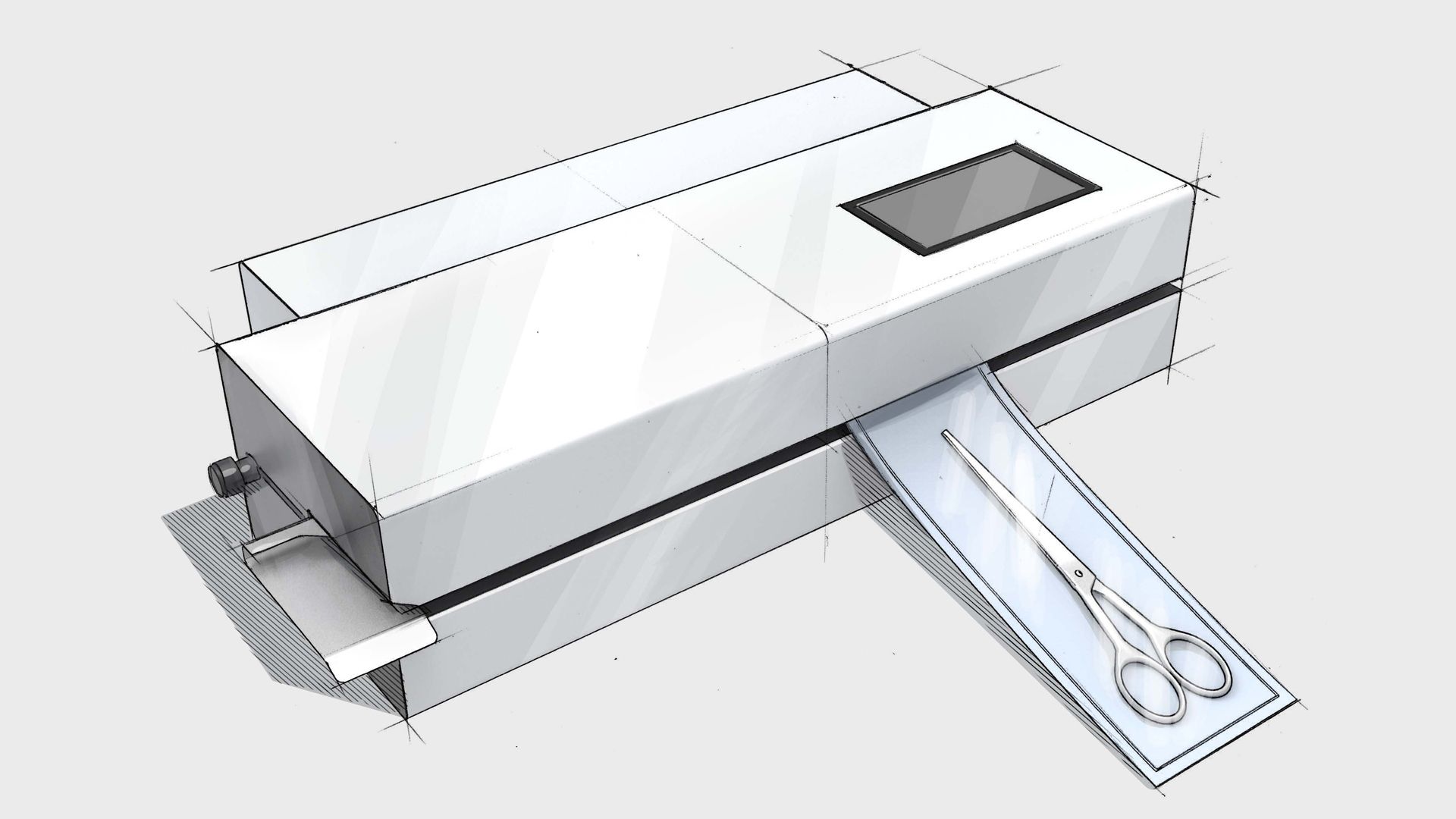
MD-880 medical device pouch sealer
Our rotary sealer allows operators to feed their medical device pouches rapidly through the feed system for an impressive throughput. The MD-880 medical device rotary sealer can also print on the Tyvek® side of your pouch with important information such as expiry dating and lot numbers. We include a calibration from our award-winning ISO 17025 calibration Laboratory as well as a host of packaging validation support.
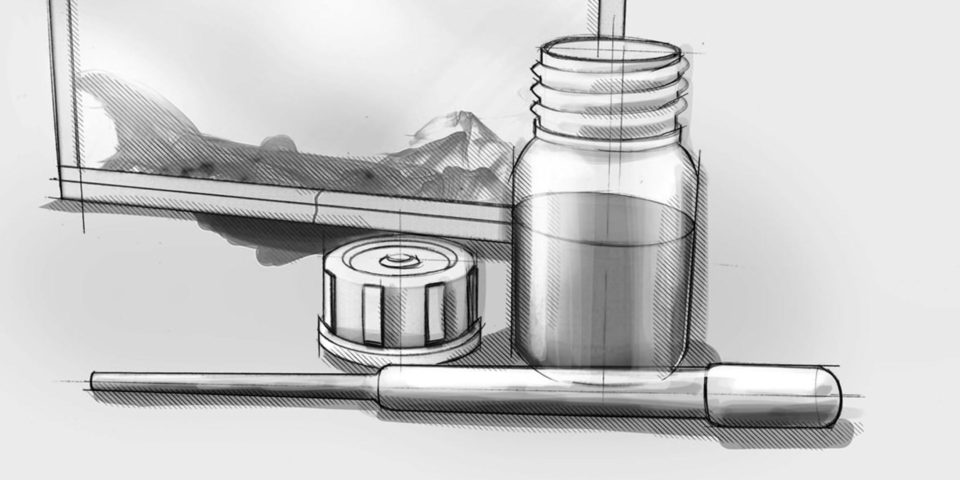
Compliant premixed dye solution
ASTM F1929-15 ready premixed medical device pouch test solution for rapid testing. Now medical device packagers can purchase our premixed ink in bulk quantities. For our customers with a more demanding workflow that requires multiple dye penetration testing events throughout the day, we now offer a premixed solution in 8oz, 16oz, as well as a 32oz sizes. The dye solution comes in FDA-compliant Boston bottles.
Safe and sterile device delivery is our promise to the patient…
SterileAware™ Certified Systems
Sterile packaging support
ISO 17025 empirical laboratory services
Let us evaluate your medical device packaging by utilizing the ASTM F-88 seal strength test as well as the ASTM F-1929-15 dye penetration test. Our ISO 17025 accredited laboratory provides you easy to interpret high-resolution reporting that provides high value to your audit pathway. Our testing technicians are quality system certified to assure the most accurate testing. Contact our engineering group today and let’s get started.
The value of accredited Calibrations
Most providers of medical device packaging machinery require you to send your machinery to a third-party lab for calibration. We support our medical device manufacturers with two ISO 17025 accredited laboratories that create a seamless connection between the machine and your regulatory needs. The SterileAware™ value means purchasing critical packaging machinery from firms that support your regulatory requirement.
More than just a mission of awareness, we bring action…
Our Story and mission for
Improved Patient safety…
We believe when a cause is fueled by determined corporate might, guided with the heart of a non-profit, great things will be born. The SterileAware™ hybrid is about bringing to the market solutions and awareness for lowering HAI’s. Observation from our founder’s hospital bed when sick with an HAI was the catalyst for creating solutions for better patient safety. Exciting things are happening as we build this platform, come, and watch as our mission grows…
A challenging journey ahead
The SterileAware™ Mission is to examine healthcare-associated infections and deliver solutions engendered by our think tank. The mission will also include a campaign of awareness to the public about the dangers of cross-contamination and sterility loss at hospitals.
The SterileAware™ Podcast goes live
The SterileAware™ Podcast will provide insight on the Hospital born infection story with interviews with leading experts in HAI’s. The SterileAware™ Podcast is hosted by our founder Charlie Webb, an award-winning podcaster and host of the popular Healthcare packaging Podcast “SPOT radio”. Stay up to date with the SterileAware™ story. Listen now
SterileAware™ on stage at the MD&M
SterileAware™ founder Charlie Webb CPPL presented the SterileAware™ story on the pack place stage at the Medical Device Manufacturing Expo this past February. Webb reported, “I was amazed with the excitement generated from my talk, so many connections asking to get involved, we are clearly on an important path”. Expect more speaking dates ahead.

Celebrating with the Superstars in Sterile Awareness™ Award
|
So, what does the selection process for the SterileAware™ ‘Superstars in Sterile Awareness award’ look like? Does it require a core educational level or baseline years in the industry or published work? The answer is none of the above. The superstars that we seek are the hard-working, big-hearted individuals that put in the extra time and effort to make a difference in patient safety.
I have been in medical device development for 30 years and in medical packaging for about 27 years. Over these decades I have connected with thousands of individuals and companies that serve the sterile device delivery space. The ‘Superstars in Sterile Awareness award’ gives me, my colleagues, and you as well the opportunity to recognize the people that are making worthwhile contributions. This includes everything from medical device manufacturers, healthcare processing and packaging professionals as well as all the healthcare workers in hospitals and clinics that support a patient’s safe journey through a healthcare facility.
Through our street-level observations, we will identify standout superstars and avoid the multi-layers of the bureaucracy of traditional Industry awards. We believe this will democratize the recognition process and give much-deserved credit to healthcare value chain superstars that may fall under the radar if not nominated under other industrial award platforms.
So, will we be awarding colleagues that we have a close relationship with that some may call “Friends”? Of course, we will however never play the cronyism game. It is very likely that the people being recognized for their outstanding service in healthcare packaging will be people that we are close to, after all, the healthcare value chain is a small ecosystem.
Can you nominate someone for the SterileAware™ ‘Superstars in Sterile Awareness award’? Absolutely! We can’t be everywhere and we’re not going to say that we know every company and person that pushes the needle forward in our industry, so if you have a candidate in mind, drop us an email we would love to hear their story… info@sterileaware.com
Sarah Rosenblum Ptach – Superstar recognition for her healthcare packaging industry omnipresence, and her tireless contribution to the KiiP group, the Pack-Out and other industry organizations.
Elon Goldbaum – Elon’s Superstar recognition comes from his leadership skills with his role at the Network Partners group and beyond. He is a go-to SME in medical device packaging.
Justin Anderson – Justin is the master of boot strap and rapid deployment and demonstrated those skills building out a cleanroom and packaging workstation for Tensor surgical.
Amy Stewart – Despite her demanding position at Printpack, Amy is deeply involved in a host of committees and groups in healthcare packaging. Her organizational skills are stellar!
Kelly Mawer – In her role as a Production Scientist with BIOLYPH Kelly closely monitors the exacting details of her work with a granular optic on healthcare packaging Quality control.
Harry Oussoren – Superstar award for his passion for device sterility. Since 2011, Harry has been President of the Dutch Society of Sterile Medical Devices Experts and Vice President and Board Member of the World Federation for Hospital Sterilization (WFHSS).
J. Darrel Hicks – He is the author of “Infection Control for Dummies” and his passion is for learning and teaching others about the importance of cleaning as it relates to the prevention of infections.
Buffy Lioyd-Krejci – Dr. Buffy Lloyd-Krejci is passionate about Infection and is one of the foremost authorities on infection prevention and control in nursing homes and long-term care facilities.
Nevoa – Superstar product award winner for infection prevention with their whole-room hypochlorous acid (HOCl) fogging technology.
David Jagrosse – A champion teacher that has created no-cost content on Linkedin to educate SPD technicians across the US. He is passionate about the safe and sterile presentation of devices in the OR.
Tyra Smith – Her heartfelt nomination described all her activities as an SPD technician. She has gone well beyond the call of duty to assure the safe and sterile delivery of medical devices.
Malinda Elammari – A true Superstar and SME regarding all functions of the SPD. She travels the country speaking on improved techniques in Sterile Processing. She is a role model fixture to our industry.
Christine Denis – Christine from the (WFHSS = World Forum For Hospital Supply) recognized for her outstanding commitment for HAI prevention and healthcare packaging.
Industry connecting to a cause
The heart of .org and the strength of a .com
At SterileAware, we want our corporate partnerships to model some of the best qualities of businesses and not-for-profit organizations. That’s why our collaboration with Van der Stähl Scientific has originated to help us expand on our mission to develop medical device packaging solutions that meet the growing demands of a rising global population. Learn More
Solutions born from collaboration
Our “innovation partners program” is an innovation incubator designed to imagine new technologies that will serve to mitigate the spread of hospital associate infections. Improved packaging and medical device pouch testing systems will be our first target as we can leverage our 27 years of experience. Stay tuned as we take aim at patient safety.
It’s time for a Road trip, we are on the road for patient safety…
At SterileAware® we will push the term “dispersed workplace” to a higher level as we weave work and our personal travel together on an epic Road-trip. We purchased a 4X4 Overland go-anywhere Camper that will serve as our mobile office and living quarters as we circumnavigate the western US. We plan on dropping into hospitals and clinics to leave behind infographic posters as well as awareness bracelets, and we hope to book a few talks along the way…